CAPA Management
Automated CAPA for Audit Readiness
Automate CAPA workflows to improve compliance, accelerate resolution timelines, and drive continuous improvement — all within one connected system.


Smarter CAPA, Proven Compliance
Streamline CAPA Workflows and Strengthen Compliance
Guide your team from issue to resolution with structured, audit-ready CAPA processes. Grand Avenue’s CAPA software helps companies reduce human error, accelerate response times, and document every step in an audit-ready format. Whether it’s preventive or corrective actions, teams can access relevant data, track progress through visualized workflows, and implement action items efficiently.
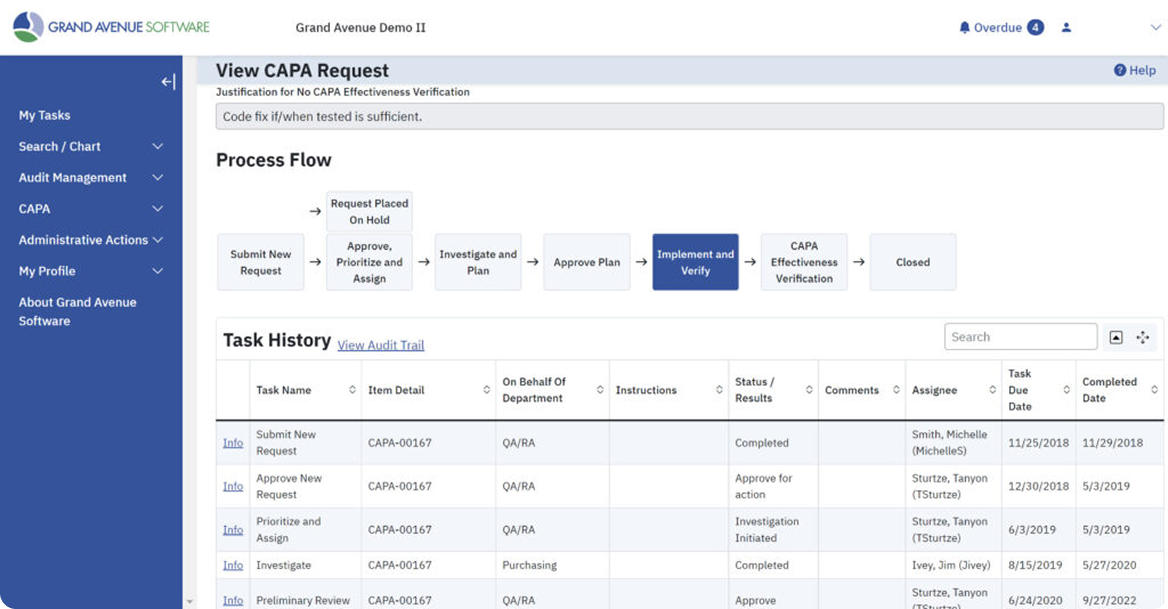
CAPA Insights That Drive Action
Visualize trends, track sources, and stay ahead of recurring issues. Grand Avenue transforms CAPA data into actionable insights with built-in charts and reporting tools. Instantly group CAPAs by source, monitor resolution trends, and identify high-risk areas — so you can focus your efforts where they’ll have the greatest impact.
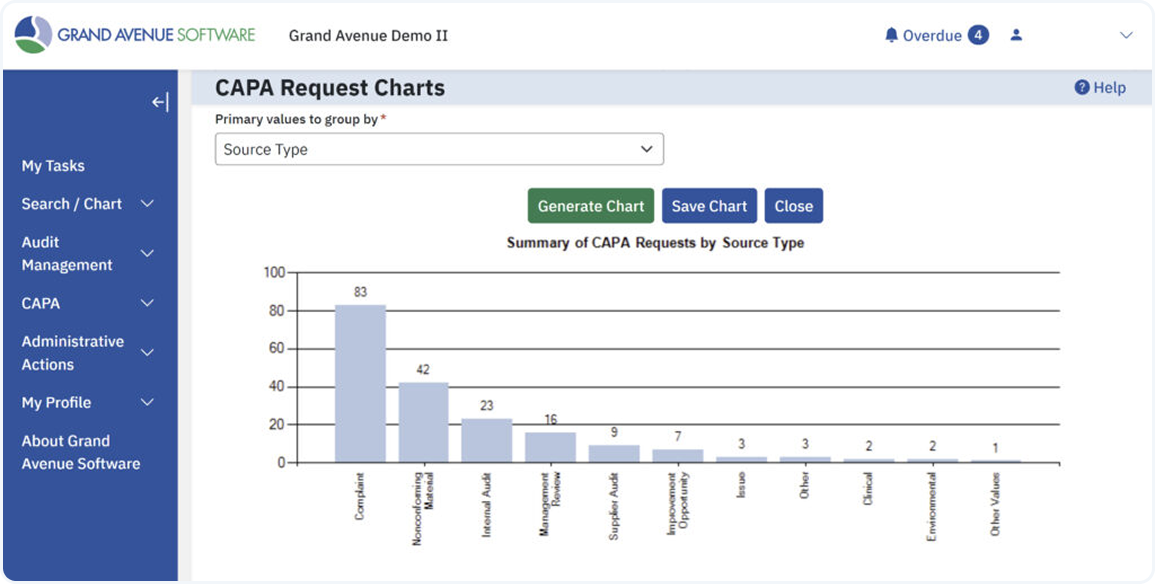
Built-In Compliance, Right Out of the Box
Reduce risk, streamline compliance, and improve quality with less effort. Grand Avenue’s CAPA solution fits into your existing workflows to help teams manage risk, resolve issues faster, and stay aligned with FDA and ISO standards. From audit trails to root cause analysis, you’ll have the tools you need to stay compliant — and efficient.
Frequently Asked Questions
Grand Avenue’s CAPA module streamlines the submission and tracking of CAPA requests by offering automated workflows that assign tasks, set deadlines, and provide real-time updates. This ensures issues are quickly addressed, tracked, and escalated when necessary, improving compliance and efficiency.
Grand Avenue verifies the effectiveness of CAPA actions by requiring follow-up tasks and effectiveness checks to ensure corrective or preventive measures successfully resolve the identified issue. These steps help ensure compliance and reduce the likelihood of recurring problems.
Grand Avenue’s CAPA module efficiently manages both corrective and preventive actions through its structured workflows. It allows you to track both types of actions, ensuring thorough resolution of issues while helping to prevent future occurrences, all while meeting FDA and ISO requirements.
What is the CAPA workflow in Grand Avenue Software, and how does it help with root-cause analysis?
Grand Avenue’s CAPA workflow helps resolve non-conformities by facilitating issue identification, root cause analysis, and corrective/preventive action implementation. It provides a structured process for investigating issues, ensuring effective resolution and compliance with regulatory standards.
Grand Avenue’s CAPA module ensures compliance with FDA and ISO 13485 by integrating corrective and preventive action (CAPA) workflows. It supports root cause analysis, assigns responsibilities, tracks corrective actions, verification of effectiveness and ensures all steps are documented, meeting regulatory requirements.